At GENOTRIX you’ll find the best practices in the market for you medical device project. Each project will have a custom-designed approach using the best acknowledged standards and GMP guidelines to fulfil your needs and to develop the best device for the market and for the patient. That is why you can rely on us to be your Contract Research Organization (CRO) and your Contract Development and Manufacturing Organization (CDMO).
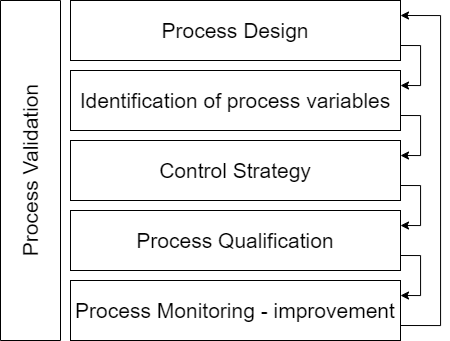
For concepts and prototyping we adopt the waterfall model to start shaping your project.
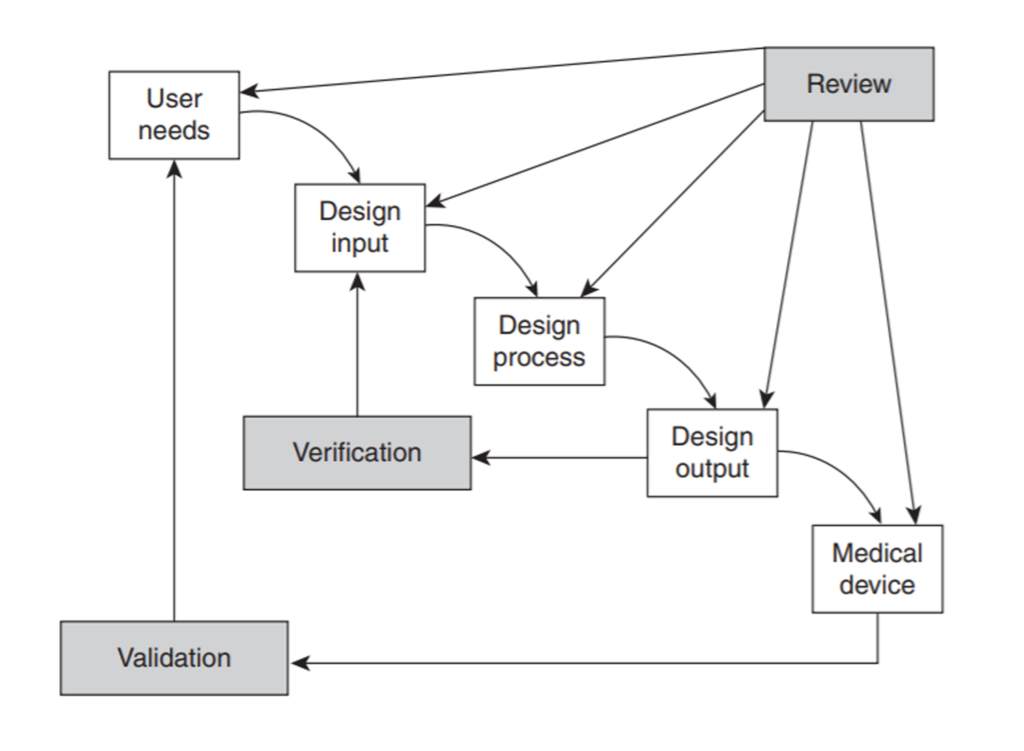
For wider projects we adopt a global perspective based on a more complete GMP model that covers each step and project stage.
Source(reference): https://doi.org/10.1533/9780857099204.115





Concept and Viability
Concept Prototyping
Development
Product Enhancement
Market Compliance
Go2Market
Full lifecycle management
Market Access roadmap
Requirements and Regulation Compliance
Market Monitoring
QMS
In the first moment we will understand the main concept in order to develop an early approach for the device specifications, usability, safety issues and other relevant information that could lead us for a feasibility study.
In this step it is fundamental to gather from market all the necessary inputs and examples for a better product placement and to identify all the potential risks involved to avoid, minimize or mitigate in the future steps.
With all the initial steps done we will start a first design proposal for the product classification and regulatory compliance with all the necessary and identified requirements.
With the first design done we will then confirm and verify all the information through a deep technical research and content review.
At this stage you will get the strategical plan for development and manufacture, as well the regulatory requirements needed for the next stages including the registration approach and all the market strategy you will need.
Our commitment to quality is present at each step, including documented validations and the relevant quality controls.
GENOTRIX will develop a proof-of-concept to your Medical Device prototype which shall be prepared for a real case scenario and ready for a first trial.
All the clinical and usabality guidelines and compliance are respected and present in this step to identify and mitigate any safety issue that could come up.
GENOTRIX will address you all the financial aspects you need for your project, as well all the evidence requirements and product evaluation for a global overview and market perspective from, targets to stakeholders.
At this point we will review the project to scale-up and the alternatives -if necessary- to adjust the processes or anything in the concept and overall idea. Everything is done with proper scientific literature from the market and from the best innovation centres and academia.
Our QMS department will confirm and audit each step and establish a first contact and connection with the Notified Body.
Your product is ready for going live and make its entrance in the market. For that you need to confirm all the safety issues, generate evidence for approval and market access.
We will develop a wide testing through tailor- and custom-made clinical trials to cover performance and safety, exceptions and other usability issues to be documented.
We will develop all the necessary documention you need about the product value to all the stakeholders. From the medical device risks and benefits, to usability, labeling, technical and comercial files, dossiers, etc. We will cover all the market and regulatory needs and best practices.
Based on the regulatory standards for similar medical devices and materials, performance, usability, manufacture and indutry protocols, guidelines and trials GENOTRIX will compile and create a product dossier for validation.
Our QMS will audit and validate the final dossier all all related procedures.
GENOTRIX will perform an additional step looking for potential enhancements and to ensure the consistence of the product and project. This step will also cover all the steps and all the dossiers made before.
Our QMS will audit and follow up this validation to guarantee all the compliance.
A new document will be made covering the main aspects of this intervention, demonstrating product value and whether there is any enhancement to be introduced in future upgrades.
We will perform a formal roadmap based on the scope, product classification, clinical evaluation and investigation, notified bodies and conformity assessment.
GENOTRIX will assemble and submit the file application will all the information collected confirming the market validation and product value.
The marking procedure will cover and evaluate the medical device technical aspects and also the QMS from GENOTRIX to confirm and validate full compliance with the regulations. At this level we will also develop a wide dossier, compiling all the relevant information on the medical device, from safety to performance, from quality to the preclinical and clinical data.
GENOTRIX will also design the PMS file for the medical device monitorization system.
Once again our QMS will perform a close follow-up for full compliance.
GENOTRIX will cover all the promotional aspects and labelling validation, covering the value proposition and the market strategy.
All the information, marketing and communication materials would be regulatory-compliant at this step and adjusted to each market.
Our QMS will keep tracking, monitoring and audit the medical device for continuous improvement and to confirm the QMS sustainability.
GENOTRIX will deploy the necessary systems to monitor the safety and product performance as well the risk and benefits. The goal is to mantain full compliance and to collect relevant data.

Lorem ipsum dolor sit amet, consectetur quam adipiscing quam elit tellus, luctus nec dapibus Vestibulum id ligula porta
Necessary cookies are absolutely essential for the website to function properly. These cookies ensure basic functionalities and security features of the website, anonymously.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features.
Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors.
Analytical cookies are used to understand how visitors interact with the website. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc.
Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. These cookies track visitors across websites and collect information to provide customized ads.
Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet.
Sign up to receive updates, promotions, and sneak peaks of upcoming products. Plus 20% off your next order.